Isothermal Modelling of Glyphosate Herbicide Adsorption Using Biochar andHumic Substances from Palm Oil Mill Waste
Abstract
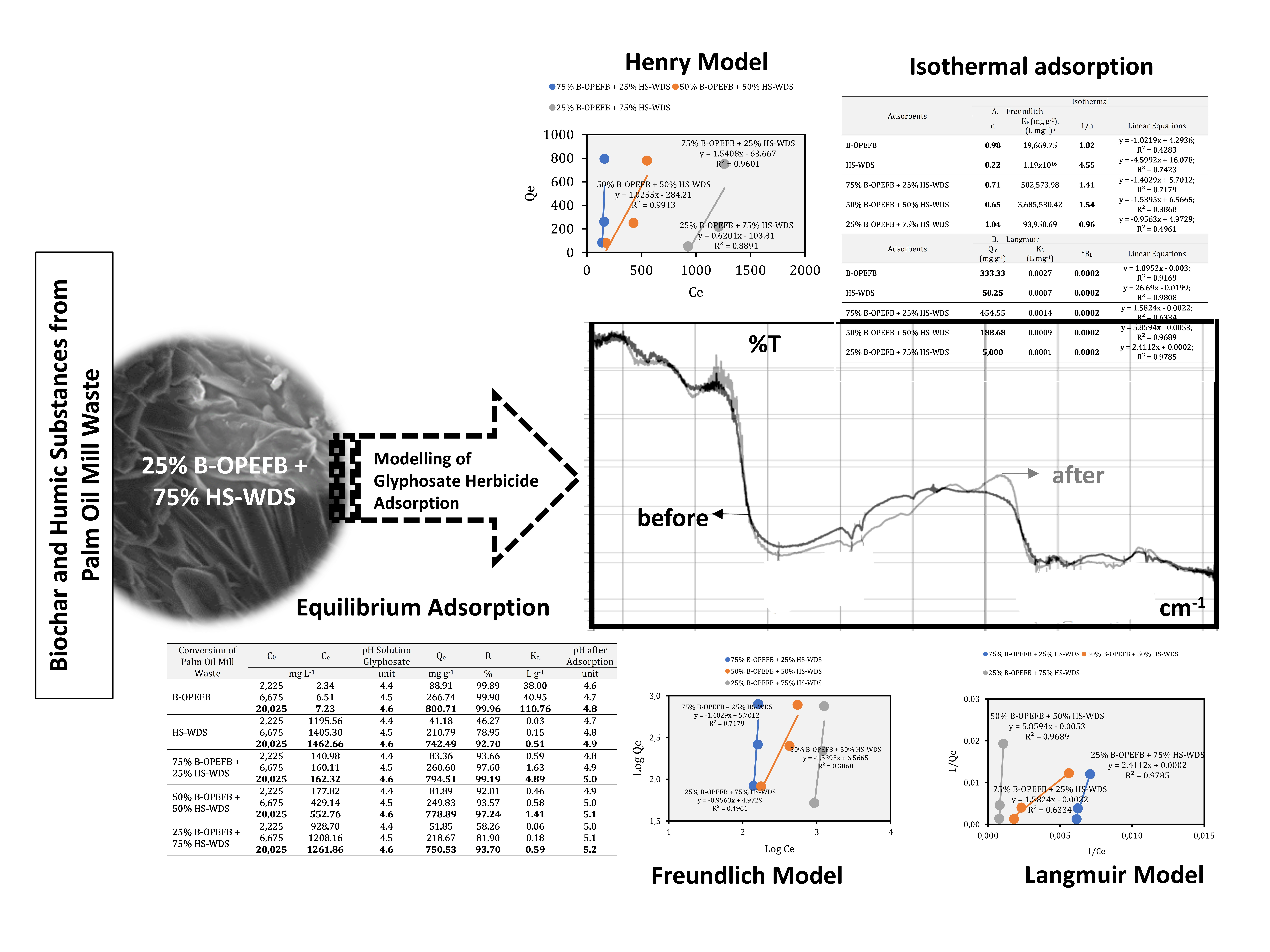
The increasing use of glyphosate containing herbicides raises environmental concerns. These herbicides are persistent environmental pollutants and may harm soil and aquatic ecosystems. Although previous studies have evaluated different adsorbents, the use of palm oil mill waste for glyphosate containment has not been thoroughly researched. This study attempts to fill this gap by turning palm oil mill waste into eco-friendly adsorbents: biochar and humic substances. This study seeks to identify the glyphosate adsorption characteristics of diverse palm oil waste formulations in order to determine the optimal formulation through an isothermal adsorption analysis. Two major materials were used in the batch equilibrium experiments: biochar created from empty palm fruit bunches (B-OPEFB) and humic substances derived from wet decanter solids (HS-WDS). Each of these adsorbents were used on their own, and in different combinations to test how well they retained glyphosate. The results revealed that the adsorption capacity of biochar is largely due to its carbonporous matrix, while humic substances contribute to the adsorption via chemical interactions that are facilitated by active functional groups. Out of all the combinations, the one containing 25% biochar and 75% humic substances achieved the best adsorption efficiencies. The adsorption behavior of this combination was best described by the Langmuir isotherm, with a strong correlation (R² > 0.97). This clearly demonstrates the effectiveness of waste palm oil mills as a soil amendment
to reduce glyphosate contamination. In addition, this waste transformation to active materials for environmental protection supports the practice of sustainable agriculture.
Full text article
References
2. Bueno de Mesquita, C.P.; Solon, A.J.; Barfield, A.; Mastrangelo, C.F.; Tubman, A.J.; Vincent, K.; Porazinska, D.L.; Hufft, R.A.; Shackelford, N.; Suding, K.N.; et al. Adverse Impacts of Roundup on Soil Bacteria, Soil Chemistry and Mycorrhizal Fungi during Restoration of a Colorado Grassland. Appl. Soil Ecol. 2023, 185, 104778, doi:10.1016/j.apsoil.2022.104778.
3. Maulana, A.; Harianti, M.; Athiyya, S.; Prasetyo, T.B.; Monikasari, M. Biochar Quality During Slow Pyrolysis from Oil Palm Empty Fruit Bunches and Its Application as Soil Ameliorant. 2025, 40, 84–96.
4. Guo, F.; Zhou, M.; Xu, J.; Fein, J.B.; Yu, Q.; Wang, Y.; Huang, Q.; Rong, X. Glyphosate Adsorption onto Kaolinite and Kaolinite-Humic Acid Composites: Experimental and Molecular Dynamics Studies. Chemosphere 2021, 263, doi:10.1016/j.chemosphere.2020.127979.
5. Alberti, G.; Amendola, V.; Pesavento, M.; Biesuz, R. Beyond the Synthesis of Novel Solid Phases: Review on Modelling of Sorption Phenomena. Coord. Chem. Rev. 2012, 256, 28–45, doi:10.1016/j.ccr.2011.08.022.
6. Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10, doi:10.1016/j.cej.2009.09.013.
7. Hamdaoui, O.; Naffrechoux, E. Modeling of Adsorption Isotherms of Phenol and Chlorophenols onto Granular Activated Carbon. Part I. Two-Parameter Models and Equations Allowing Determination of Thermodynamic Parameters. J. Hazard. Mater. 2007, 147, 381–394, doi:10.1016/j.jhazmat.2007.01.021.
8. Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, doi:10.1155/2017/3039817.
9. Ayawei, N.; Angaye, S.S.; Wankasi, D.; Dikio, E.D. Synthesis, Characterization and Application of Mg/Al Layered Double Hydroxide for the Degradation of Congo Red in Aqueous Solution. Open J. Phys. Chem. 2015, 05, 56–70, doi:10.4236/ojpc.2015.53007.
10. Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, doi:10.1016/j.jhazmat.2020.122383.
11. Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, doi:10.1016/j.fochx.2021.100168.
12. Tiwari, A.K.; Jha, S.; Singh, A.K.; Mishra, S.K.; Pathak, A.K.; Ojha, R.P.; Yadav, R.S.; Dikshit, A. Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry. Crystals 2022, 12, doi:10.3390/cryst12081063.
13. Lin, H.; Xie, J.; Dong, Y.; Liu, J.; Meng, K.; Jin, Q. A Complete Review on the Surface Functional Groups in Pyrolyzed Biochar and Its Interaction Mechanism with Heavy Metal in Water. J. Environ. Chem. Eng. 2025, 13, doi:10.1016/j.jece.2025.116681.
14. Odoemelam, S.A.; Oji, E.O.; Eddy, N.O.; Garg, R.; Garg, R.; Islam, S.; Khan, M.A.; Khan, N.A.; Zahmatkesh, S. Zinc Oxide Nanoparticles Adsorb Emerging Pollutants (Glyphosate Pesticide) from Aqueous Solutions. Environ. Monit. Assess. 2023, 195, doi:10.1007/s10661-023-11255-0.
15. Lyu, H.; Zhang, Q.; Shen, B. Application of Biochar and Its Composites in Catalysis. Chemosphere 2020, 240, doi:10.1016/j.chemosphere.2019.124842.
16. Dai, L.; Lu, Q.; Zhou, H.; Shen, F.; Liu, Z.; Zhu, W.; Huang, H. Tuning Oxygenated Functional Groups on Biochar for Water Pollution Control: A Critical Review. J. Hazard. Mater. 2021, 420, doi:10.1016/j.jhazmat.2021.126547.
17. Alves, P.A. de T.; Munhoz-Garcia, G.V.; Nalin, D.; Mendes, K.F.; Tornisielo, V.L. Understanding the Complexities in Glyphosate and Ametryn Interactions: Soil Retention and Transformation as Influenced by Their Applications Alone and Mixture. Crop Prot. 2024, 184, doi:10.1016/j.cropro.2024.106803.
18. Herviyanti; Maulana, A.; Prasetyo, T.B.; Lita, A.L.; Harianti, M.; Monikasari, M. Characteristics of Glyphosate Adsorption with Biochar from Young Coconut Waste. IOP Conf. Ser. Earth Environ. Sci. 2023, 1208, doi:10.1088/1755-1315/1208/1/012050.
19. Kimbi Yaah, V.B.; Ahmadi, S.; Quimbayo M, J.; Morales-Torres, S.; Ojala, S. Recent Technologies for Glyphosate Removal from Aqueous Environment: A Critical Review. Environ. Res. 2024, 240, 117477, doi:https://doi.org/10.1016/j.envres.2023.117477.
20. Sen, K.; Chattoraj, S. 5 - A Comprehensive Review of Glyphosate Adsorption with Factors Influencing Mechanism: Kinetics, Isotherms, Thermodynamics Study. In Intelligent Data-Centric Systems; Bhattacharyya, S., Mondal, N.K., Platos, J., Snášel, V., Krömer, P.B.T.-I.E.D.M. for P.M., Eds.; Academic Press, 2021; pp. 93–125 ISBN 978-0-12-819671-7.
21. Sun, S.-J.; Wang, F.; He, Z.-W.; Tang, C.-C.; Zhou, A.-J.; Ren, Y.-X.; Li, Z.; Liu, W. Biochar Alleviates Inhibition Effects of Humic Acid on Anaerobic Digestion: Insights to Performances and Mechanisms. Environ. Res. 2024, 259, 119537, doi:https://doi.org/10.1016/j.envres.2024.119537.
22. Wei, J.; Yan, L.; Zhang, Z.; Hu, B.; Gui, W.; Cui, Y. Carbon Nanotube/Chitosan Hydrogel for Adsorption of Acid Red 73 in Aqueous and Soil Environments. BMC Chem. 2023, 17, 104, doi:10.1186/s13065-023-01019-9.
Authors
Copyright (c) 2025 Salma Athiyya, Yulnafatmawita, Amsar Maulana, Herviyanti

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).